What are multi walled carbon nanotubes? MWCNT production, properties, and applications
Carbon naturally occurs in several solid forms, known as allotropes. You may be familiar with some of these, or even have them in your home, such as coal, diamonds, and graphite (the “lead” in a standard pencil). They are all formed from carbon atoms, but those atoms are assembled in different ways, which gives rise to their different properties, such as graphite’s softness and diamond’s sparkle.
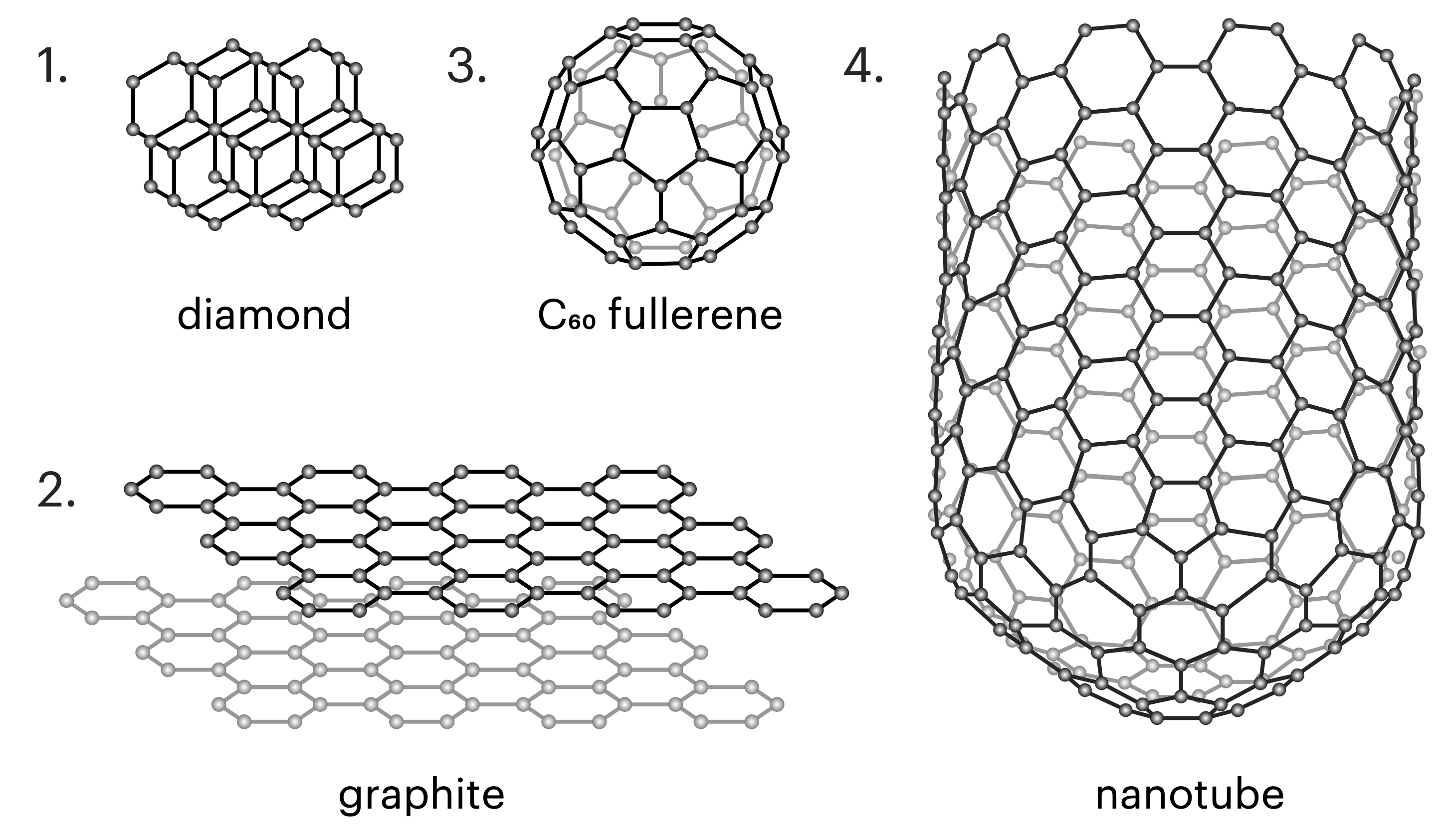
Even more forms of carbon have been synthesized in the lab over the years, including graphene (sheets made of a single layer of carbon atoms) and carbon nanotubes. Like graphene, these nanotubes are made of carbon atoms in a lattice just one atom thick, but instead of being arranged in a sheet, they form long, hollow tubes.
Graphene nanotube 3D model
In the past, most commercial applications dealt with multiwall carbon nanotubes (MWCNTs), which can be thought of as a number of tubes nested inside each other, a bit like the rings of a tree or a folded telescopic antenna, as shown in Fig.2. The production technology of MWCNT is relatively unsophisticated and has been successfully developed by over 120 companies around the world 1.
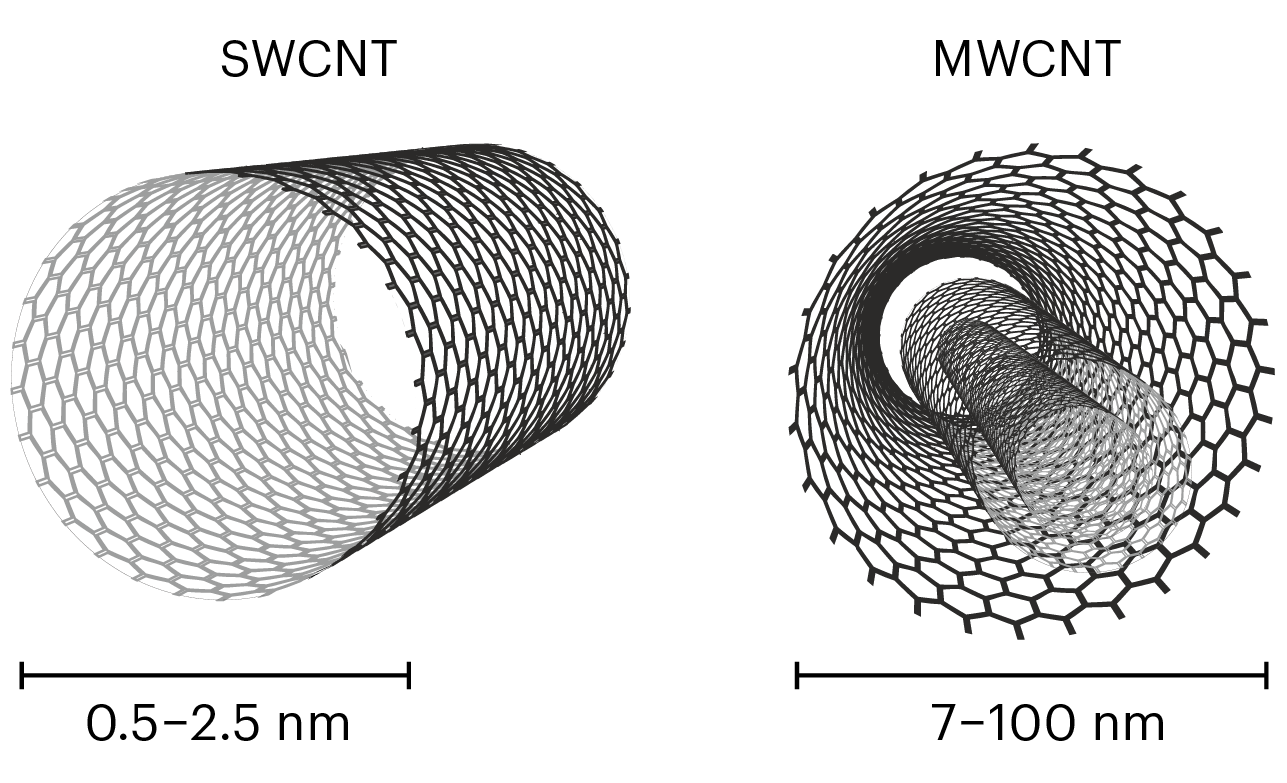
The synthesis technology of nanotubes with a wall only one layer thick, or single wall carbon nanotubes (SWCNTs), is more complicated. So far, only one manufacturer of single-walled carbon nanotubes has succeeded in large-scale production and thus is dominating the market: OCSiAl with its TUBALLTM nanotubes.
MWCNT properties
Physical properties of MWCNTs
We said above that MWCNTs are long tubes, but the length itself is not the crucial characteristic in many applications. The aspect ratio, i.e., the ratio of the length to the diameter, is often more important. For MWCNTs with typical diameters between 7 and 100 nm, the aspect ratio is typically between 50 and 4,000. With just single-layer walls, SWCNTs are even thinner (0.5 to 2.5 nm) and because of that their aspect ratio is typically greater and often goes up to 10,000.
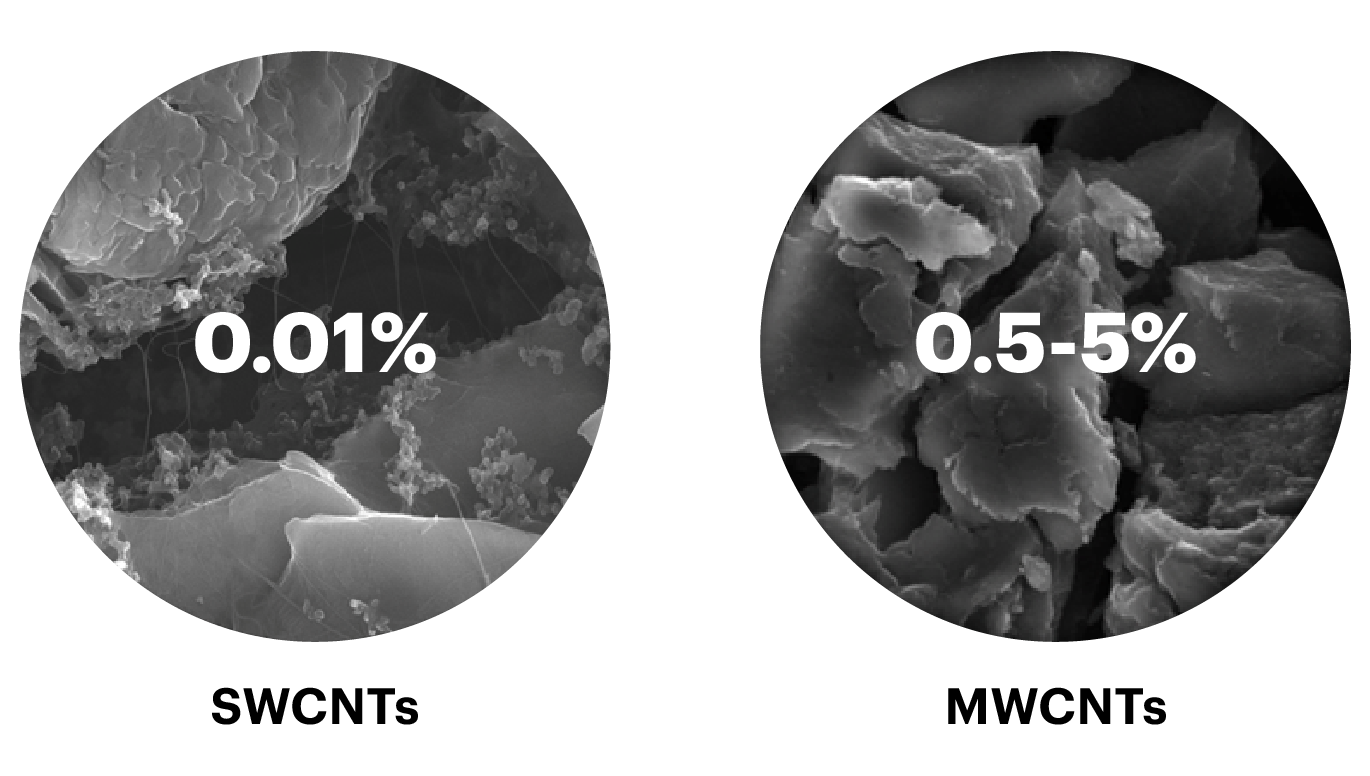
These ratios have a significant impact on the performance of MWCNTs and SWCNTs as additives in other materials such as various plastics.
Electrical conductivity of multi-walled carbon nanotubes
One of the most advantageous properties of carbon nanotubes is their high electrical conductivity. The beauty is that when even relatively small amounts of MWCNTs are mixed into normally insulating materials, the materials can gain significant conductivity. Applications include things like conductive latex gloves for touchscreen use or antistatic conveyor rollers, which can dissipate the static charge.
While traditional conductive additives such as carbon black can also impart conductivity, the length-to-diameter ratio of multi wall carbon nanotubes makes it possible to achieve that at much smaller amounts, because the long tubes can contact each other over longer distances and form a conductive network through the material. SWCNTs can achieve the same effect at even smaller concentrations. Because of the thinner walls (greater aspect ratio), the same conductive network can be built from a smaller mass of the additive.
The greater flexibility of SWCNTs compared to MWCNTs is an additional helpful feature here as well. As you might expect, the many concentric layers in the wall of an MWCNT make it not only thick but also stiff, while an SWCNT with its single-layer wall is highly flexible. As a matter of fact, it is more flexible for smaller diameters.
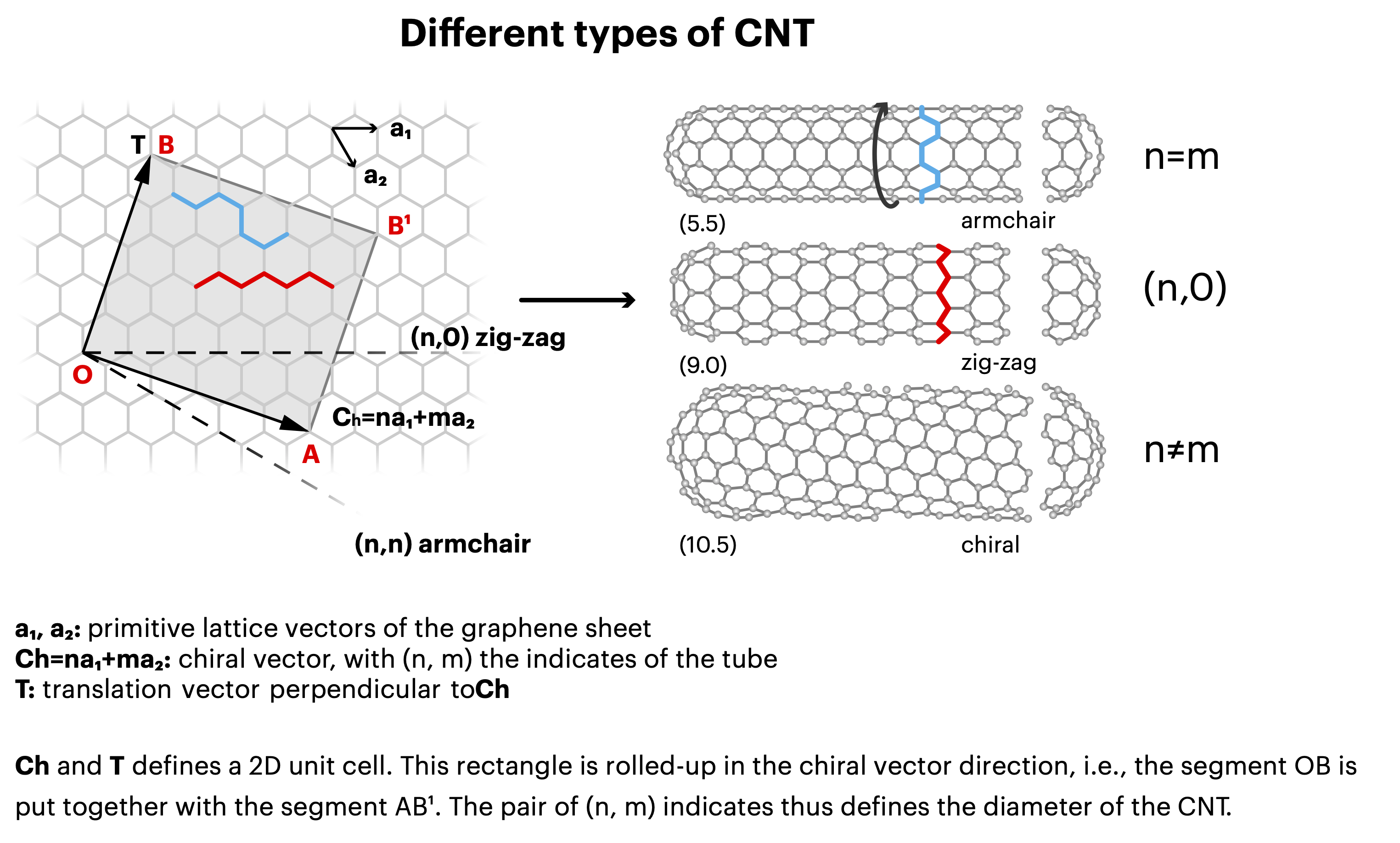
There is one caveat though that makes the effect often less pronounced than it could be, because not all SWCNTs conduct electricity with the same efficiency. Carbon nanotubes can ‘grow’ in a different pattern, as illustrated in Figure 4. Although each tube has the same ingredient (carbon atoms arranged in a hexagonal lattice), the level of conductivity varies with chirality (the angle at which this carbon sheet is oriented with respect to the tube’s axis). It ranges from metallic (i.e., excellent) for “armchair” tubes, to only matching that of a semiconductor on par with silicon in the case of tubes with a “zigzag” or “chiral” pattern. Statistically, semiconducting SWNTCs are twice as more frequent compared to metallic ones. Growing SWCNTs of a unique desired chirality thus far has been realized for a few cases in laboratories but not industrially.
Thermal conductivity of multi-walled carbon nanotubes
MWCNTs also have high thermal conductivity and can be used in normally insulative materials to increase their ability to transmit heat. This can be useful in applications where heat needs to be dissipated, such as in electronics. In cases where the use of metals is prohibited due to unwanted stresses or chemical instability, thermally conductive ceramics can be advantageous. Again, SWCNTs can achieve a similar effect to traditional conductive additives but at lower dosages, minimizing the possible negative effect on other properties of materials.
MWCNT Strength
Added into materials, carbon nanotubes can improve various mechanical properties. In tension, MWCNTs are stronger than steel, with a tensile strength of 10 to 50 GPa compared to 0.5 GPa for mild steel. When MWCNTs are used as an additive, even a small fraction inside a material can significantly strengthen it. SWCNTs are even more impressive, with a tensile strength of 50 to 100 GPa.
Multi walled carbon nanotubes production methods
Different methods have been used for the production of MWCNTs. The first method demonstrated in the laboratory was “arc discharge”. When a spark arcs in a gap between two graphite electrodes under high voltage, the resulting heat causes carbon in the positive electrode to sublimate and then condense on the negative electrode in the form of CNTs 3,4.
In another approach, “laser ablation,” a pulsed laser is used to vaporize the carbon from a graphite target. Some of the vaporized carbon forms into nanotubes when it condenses and added metal catalyst particles make it more effective. Most of the nanotubes made in this way are SWCNTs.
For larger-scale production of MWCNTs, the “chemical vapor deposition” (CVD) method is more effective, where a hydrocarbon gas is used as the carbon source in high-temperature decomposition into CNTs. This allows for greater yields and purity and thus reduces the cost of purification. The latter cannot be eliminated for the arc discharge and laser ablation methods. SWCNTs can also be produced by CVD, but the conditions including the temperature range, the hydrocarbon source, the catalyst, etc. have to be more carefully identified and controlled 5.
Applications of MWCNTs
There are many existing and potential practical applications for carbon nanotubes, where the nanotubes are introduced into materials to enhance existing properties or to provide new functionality.
As discussed above, CNTs have a suite of useful properties, which makes them an effective additive to achieve various beneficial new properties in numerous materials. For example, MWCNTs added into plastic in sufficient quantities increase their electrical conductivity. SWCNTs do it even better. This can be applied to materials other than plastics: CNTs have applications as additives in conductive coatings, conductive ink for printing electronic components, and in creating transparent, conductive films for touchscreens.
In vehicle manufacturing, carbon nanotubes can be added to make components stronger, or to achieve the same strength with less material, thereby reducing weight. Examples include carbon-fiber bicycle parts, where carbon nanotubes added to the resin improves the strength-to-weight ratio. Carbon nanotubes are also used in the manufacture of sporting goods such as tennis racquets and golf clubs.
In some cases, a combination of properties gives a new functionality. For example, silicon anodes for Li-ion batteries can be reinforced with CNTs and their resistance reduced, making them resilient to the effects of cracking during hundreds and thousands of charge/discharge cycles. Again, SWCNTs do this even better than MWCNTs. The single-walled carbon nanotubes, which have high tensile strength and flexibility, “bridge” particles together and minimize cracking.
Health & Safety aspects 5,6.
Care must be taken with handling and processing MWCNTs and MWCNT-bearing substances such as additives. Animal studies have shown that if inhaled, MWCNTs can not only enter the lungs, but also circulate to other vital organs 7. Not only that, but even a one-time exposure to an aerosol of MWCNTs can have a toxic impact on the body 8.
In this regard, it is important to reiterate that different CNTs have different physical properties.
One of the aspects of asbestos that’s relevant for its effect on the body is that the fibers are stiff. MWCNTs are more similar in this regard (as mentioned above, their multi-layer walls make them relatively stiff) than SWCNTs, which are more flexible.
- The Global Market for Multi-Walled Carbon Nanotubes 2021–2031, Future Markets. https://www.researchandmarkets.com/reports/5324906/the-global-market-for-multi-walled-carbon
- Electrical properties of nanotubes, UnderstandingNano, https://www.understandingnano.com/electrical-properties-carbon-nanotubes.html
- Jabeen, S., Kausar, A., Muhammad, B., Gul, S.,& Farooq, M., (2015) A Review on Polymeric Nanocomposites of Nanodiamond Carbon Nanotube, and Nanobifiller: Structure, Preparation and Properties, Polymer-Plastic Technology and Engineering, 54:13, 1379-1409, http://dx.doi.org/10.1080/03602559.2015.102148
- Carbon Nanotubes Market – Growth, Trends, Covid-19 Impact, and Forecasts (2021–2026), Mordor Intelligence, https://www.mordorintelligence... Nanomaterials, No. 68 (2016) Multi-Walled Carbon Nanotubes (MWCNT). ENV/JM/MONO(2016)20.https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2016)20&doclanguage=en
- OECD Environment, Health and Safety Publications Series on the Safety of Manufactured Nanomaterials, No. 70 (2016) Single-Walled Carbon Nanotubes (SWCNT). ENV/JM/MONO(2016)22. https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2016)22&doclanguage=en
- Mercer, R.R., Scabilloni, J.F., Hubbs, A.F., et al., Extrapulmonary Transport of MWCNT Following Inhalation Exposure. Part Fibre Toxicol 10, 38 (2013). https://doi.org/10.1186/1743-8977-10-38
- Francis, A.P., Ganapathy, S., Palla, V.R., Murthy, P.B., Ramaprabhu, S., Devasena, T., One Time Nose-Only Inhalation of MWCNTs: Exploring the Mechanism of Toxicity by Intermittent Sacrifice in Wistar Rats, Toxicology Reports, Volume 2, 2015, Pages 111–120, ISSN 2214-7500, https://doi.org/10.1016/j.toxrep.2015.02.00
- Francis, A.P., Ganapathy, S., Palla, V.R., Murthy, P.B., Ramaprabhu, S., Devasena, T., One Time Nose-Only Inhalation of MWCNTs: Exploring the Mechanism of Toxicity by Intermittent Sacrifice in Wistar Rats, Toxicology Reports, Volume 2, 2015, Pages 111–120, ISSN 2214-7500, https://doi.org/10.1016/j.toxrep.2015.02.003